Healon®Ophthalmic Viscoelastic Substance(眼粘弾剤)

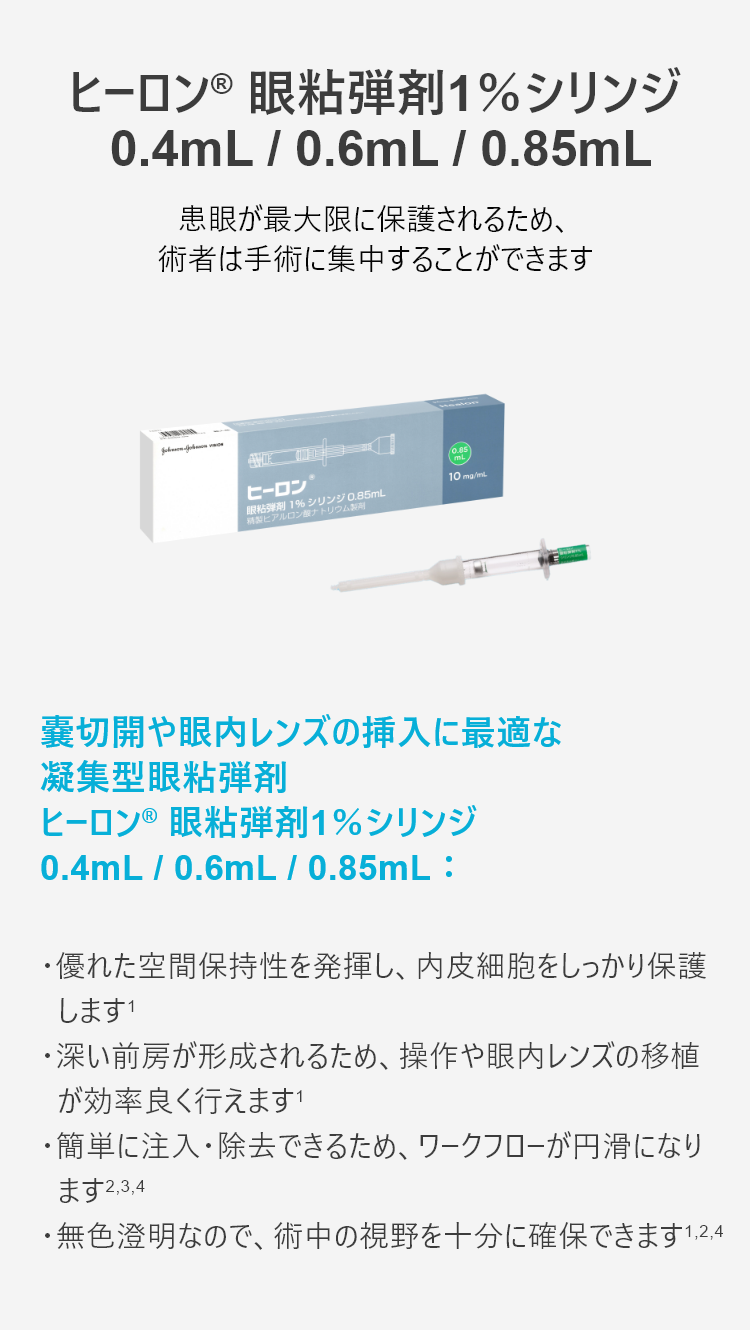
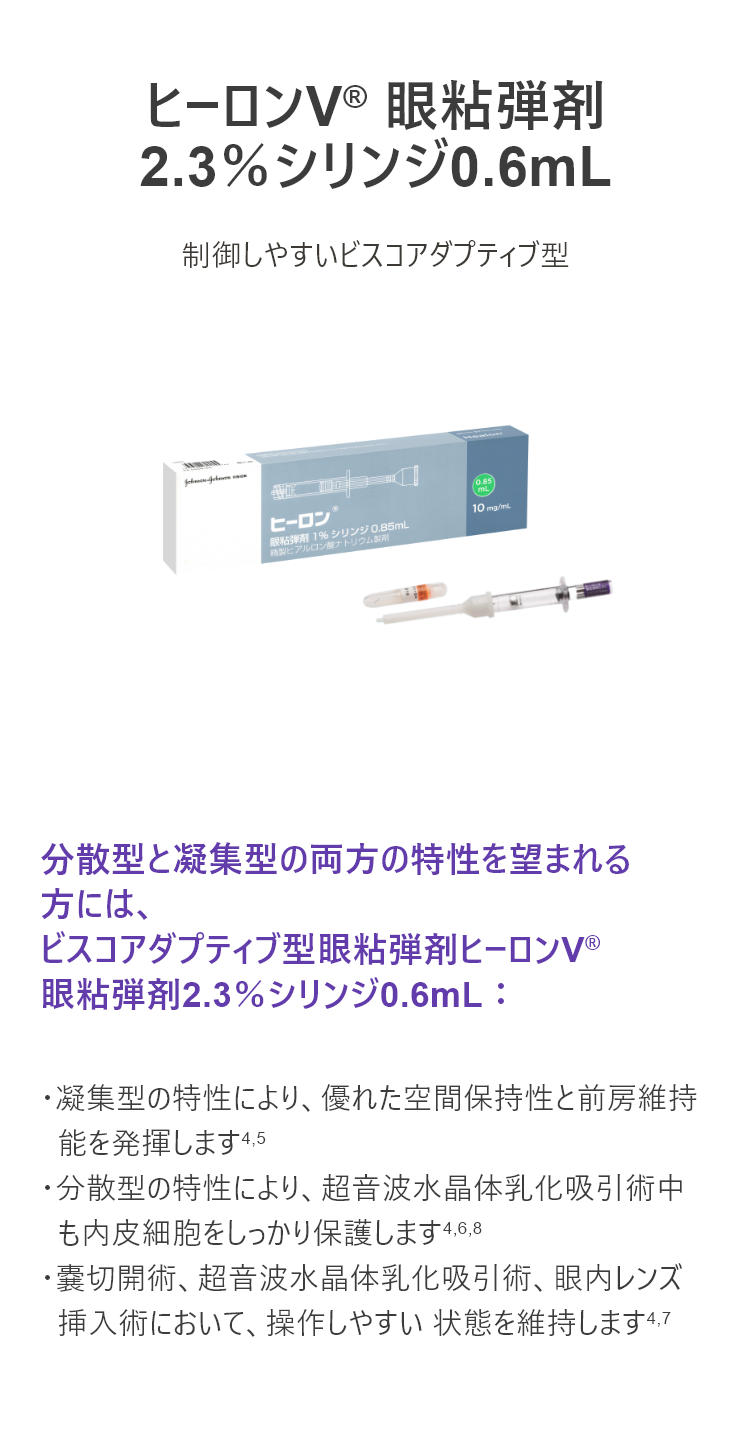
ヒーロンV® 眼粘弾剤2.3%シリンジ0.6mL
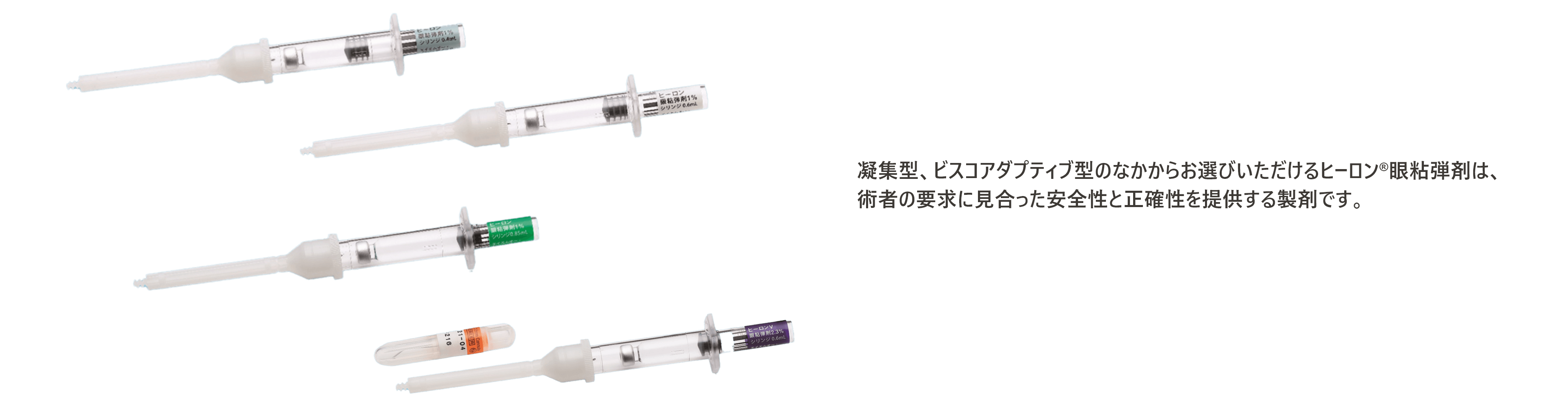
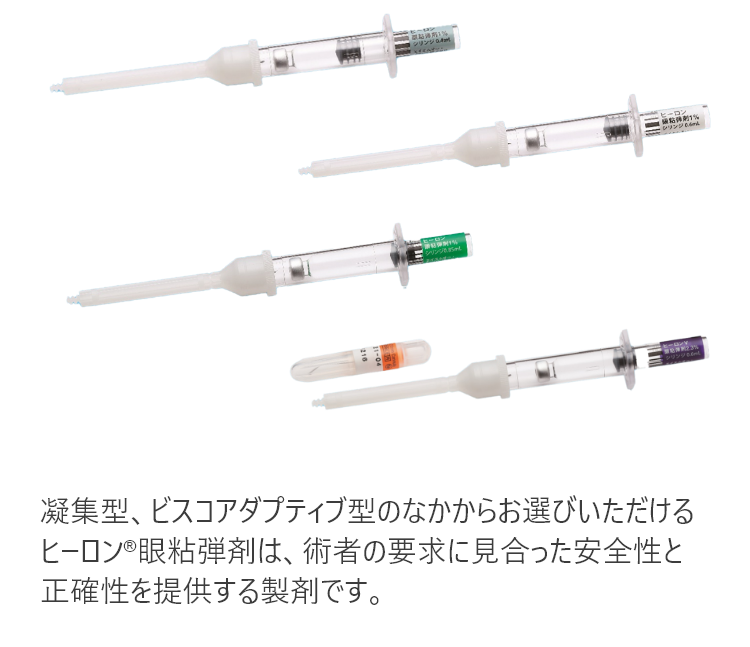
制御しやすいビスコアダプティブ型

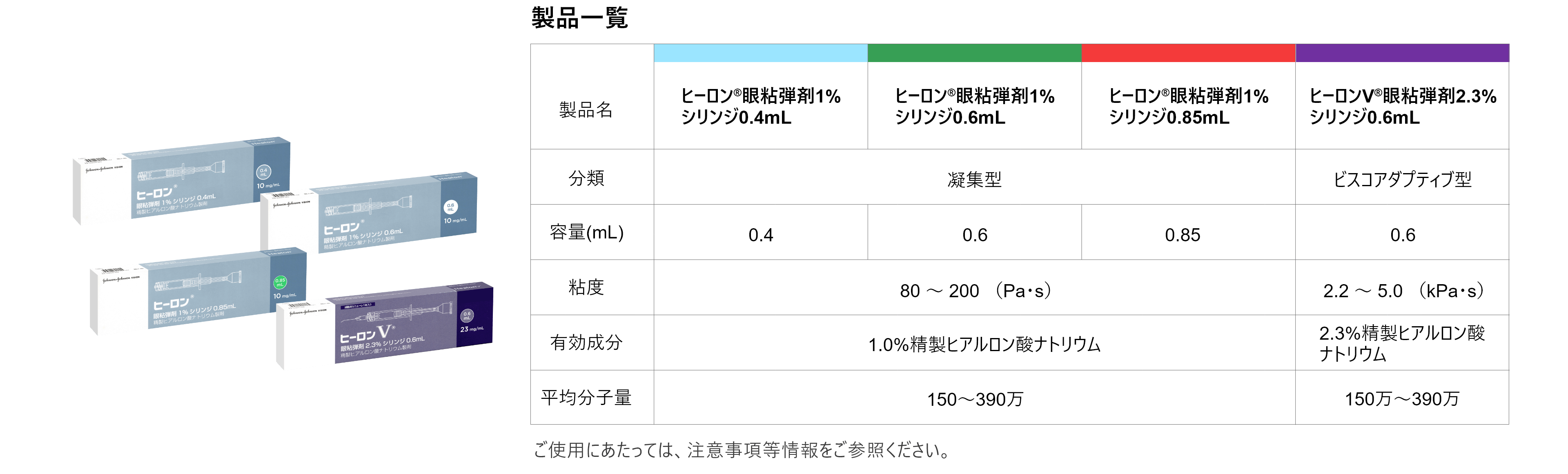

References:
1. HEALON®PRO Cohesive OVD [package insert]. Santa Ana, CA: Johnson & Johnson Surgical Vision, Inc.
2. Hutz WW. Eckhardt B, Kohnen T. Comparison of viscoelastic substances used in phacoemulsification. J Cataract Refract Surg. 1996:22:955-959
3. Bissen-Miyajima H. In vitro behavior of ophthalmic viscosurgical devices during phacoemulsification. J Cataract Refract Surg. 2006:32;1026-1031.
4. Data on File. Johnson & Johnson surgical Vision, Inc.
5. Holmen, Jorgen B. Scheimpflug photography study of ophthalmic viscosurgical devices during simulated cataract surgery. J Cataract Refract Surg 2003:29(3)568-574.
6. Holzer MP, et al. Effect of Healon5 and 4 other viscoelastic substances on intraocular pressure and endothelium after cataract surgery. J Cataract Refract Surg 2001: 27(2):213-218.
7. Tetz MR, et al. Clinical results of phacoemulsification with the use of HEALON5 or VISCOAT J Cataract Refract Surg 2001: 27(3):416-420.
8. Data on File - Clinical investigation of the bacterially-derived Healon5 OVD.
全製品ともラテックス不使用
安全性と正確性を提供